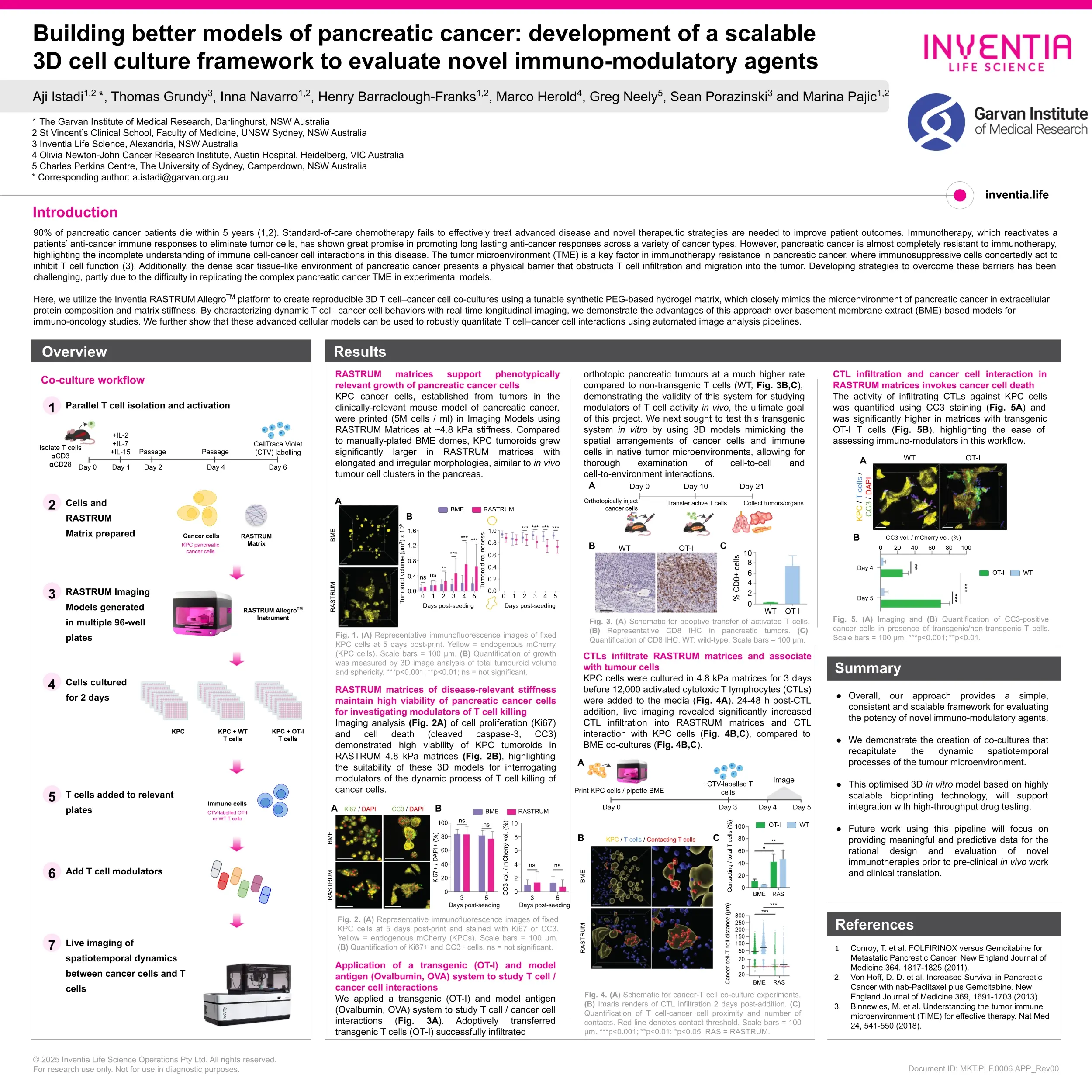
Building better models of pancreatic cancer: development of a scalable 3D cell culture framework to evaluate novel immuno-modulatory agents
Presented at Society for Laboratory Automation and Screening (SLAS) 2025
Abstract
This study aimed to develop new, biologically-relevant 3D cell culture models to develop innovative treatments to overcome therapy failure in pancreatic cancer.
Pancreatic cancer (PC) is a lethal cancer, with ~90% of patients dying within 5 years [1,2]. This dire statistic underscores the limitations of current treatments and the need for improved therapeutic approaches. Immunotherapy, which reactivates a patients’ anti-cancer immune responses to eliminate tumor cells, has shown great promise in promoting long lasting anti-cancer responses across a variety of cancer types. However, PC is almost completely resistant to immunotherapy, highlighting the incomplete understanding of immune cell-cancer cell interactions in this disease.
The tumor microenvironment is a key factor in immunotherapy resistance in PC, where immunosuppressive cells concertedly act to inhibit T cell function [3]. Additionally, the dense scar tissue-like environment of PC presents a physical barrier that obstructs T cell infiltration and migration into the tumor. Developing strategies to overcome these barriers has been challenging, partly due to the difficulty in replicating the complex PC tumor microenvironment in experimental models. The use of 3D cancer tumoroid cultures enables accurate modeling of spatial arrangements of cancer cells and immune cells, mimicking native tumor microenvironments, allowing for thorough examination of cell-to-cell and cell-to-environment interactions. However, most tumoroid cultures rely on the use of commercial matrix products like basement membrane extracts (BME), which have very limited ability to recapitulate the distinct biochemical and mechanical properties native to pancreatic tumors.
Here, we utilize the Inventia RASTRUMTM platform to create reproducible 3D T cell–cancer cell co-cultures using a tunable synthetic PEG-based hydrogel matrix, which closely mimics the microenvironment of PC in extracellular protein composition and matrix stiffness. By characterizing dynamic T cell–cancer cell behaviors with real-time longitudinal imaging, we demonstrate the advantages of this approach over BME-based models for immuno-oncology studies. Using automated image analysis pipelines we show these advanced cellular models can be used to robustly quantitate T cell–cancer cell interactions and drugs that modulate this complex interplay.
Overall, our approach provides a simple, consistent and scalable framework for evaluating the potency of novel immunomodulatory agents. Future work using this platform will focus on providing meaningful and predictive data for the rational design and evaluation of novel immunotherapies prior to pre-clinical in vivo work and clinical translation.
References
- Conroy, T. et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. New England Journal of Medicine 364, 1817-1825 (2011).
- Von Hoff, D. D. et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. New England Journal of Medicine 369, 1691-1703 (2013).
- Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24, 541-550 (2018).