One platform. Unlimited possibilities.
We develop solutions to create advanced cell models and help forward-thinking pioneers in the fields of drug discovery and biomedical research to create human tissue for research and therapy.
Our machines create advanced cell models which most closely and predictably imitate real human tissue structure and behaviour across a range of disease states. This physiological complexity offers an environment in which world-class research and discovery can occur, right in your own lab.

Our models are built for 3D cell biology, accelerating drug discovery and biomedical research.
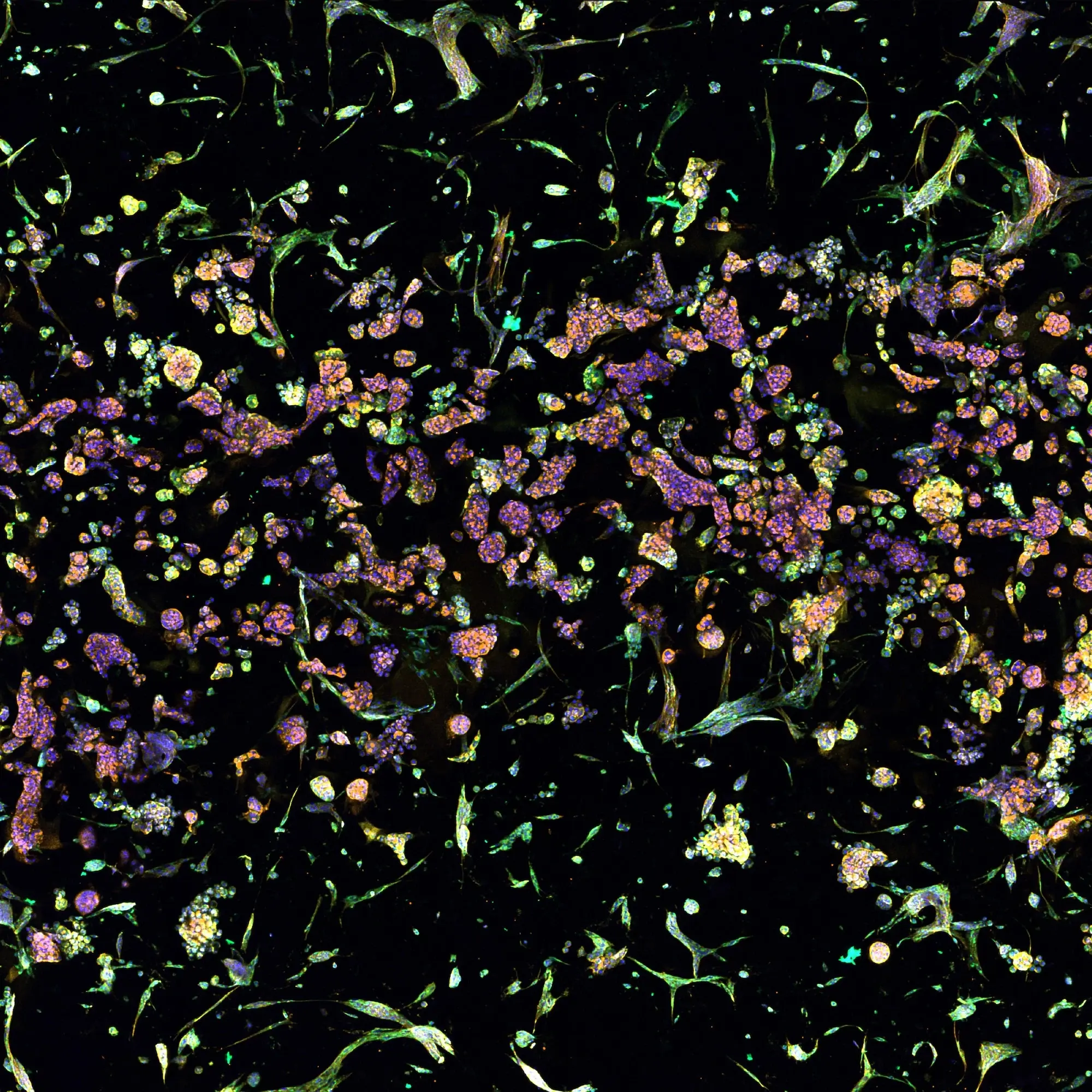
Cancer research encompasses a broad and dynamic field dedicated to understanding the mechanisms of cancer development, progression, and treatment. Cancer research faces significant challenges due to the unique nature of each cancer, requiring biologically-relevant models for developing effective therapies.
Cancer cell models generated by RASTRUM™ have made impact in key focus areas such as:
- Tumour microenvironment
- Cancer drug discovery
- Immuno-oncology
- Tumour cell migration
- Invasion
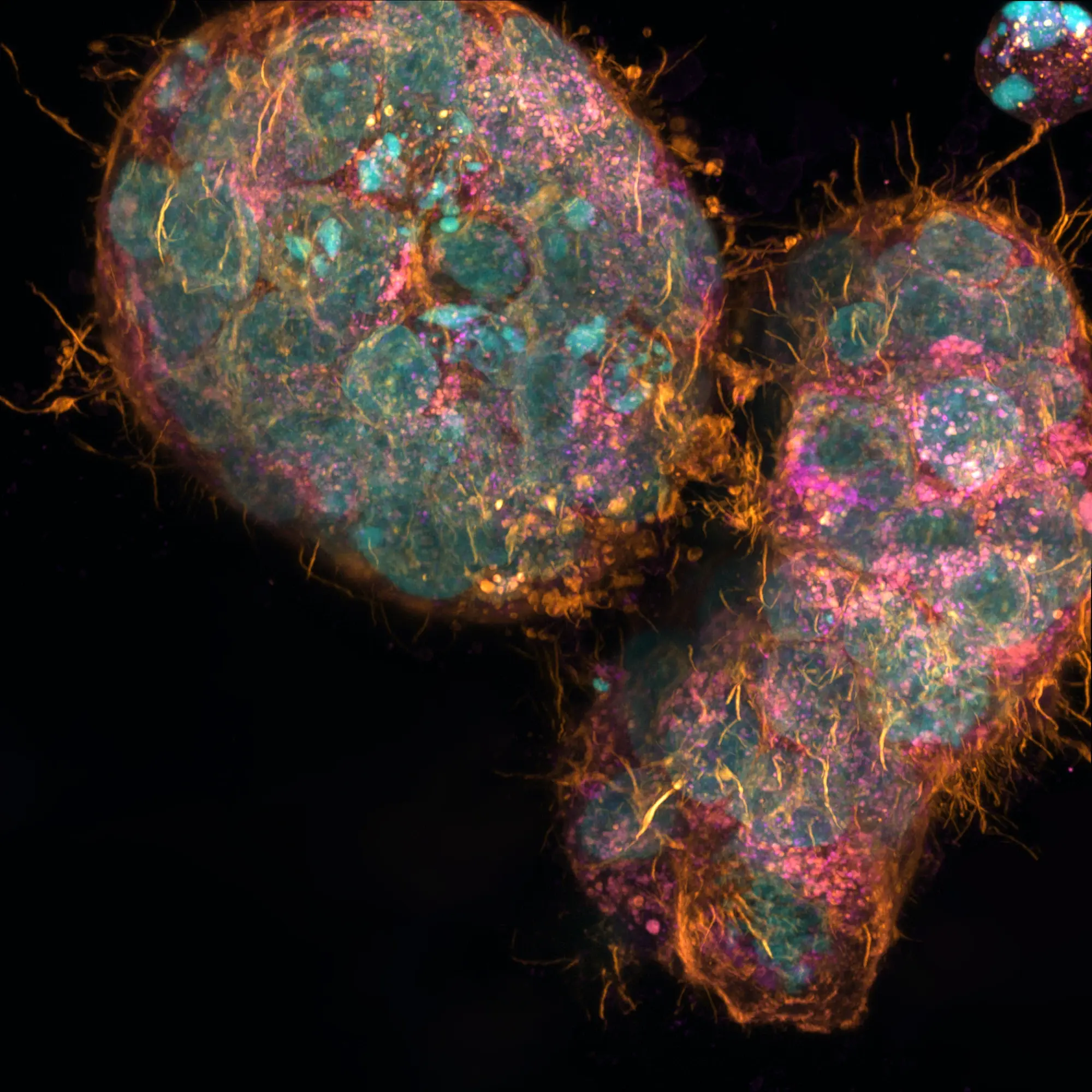
Hepatotoxicity, a leading cause of drug failure and withdrawals in clinical trials, primarily affects the liver. Animal models are unreliable for predicting human drug-induced liver injury (DILI), and current 3D cell models face challenges like poor scalability, manual handling, and workflow incompatibility.
The industry requires improved in vitro human cell models for DILI. Advanced liver cell models generated by RASTRUM™ have powerful applications for DILI studies in the preclinical phase of drug discovery.
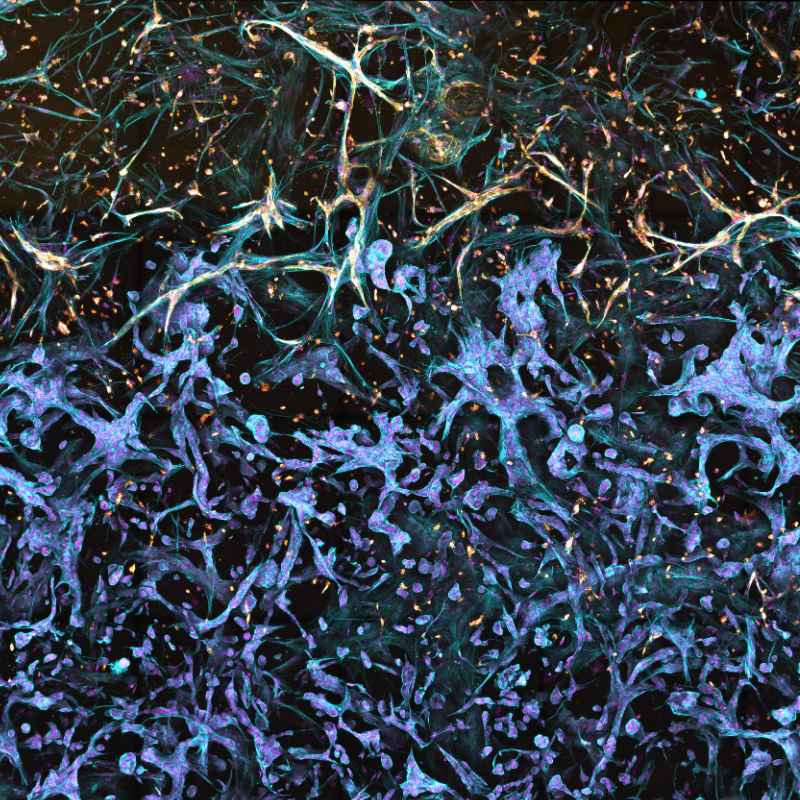
Induced pluripotent stem cells (iPSCs) offer great potential for neurodegenerative disease research and drug discovery. They can become various central nervous system (CNS) cell types, aiding the study of diseases like Alzheimer's, Parkinson's, and Huntington's. Creating physiologically relevant in vitro CNS models is vital for understanding disease and testing potential treatments.
These models reveal disease progression at a cellular level, potentially identifying unique phenotypes and therapeutic targets. Success relies on supporting multiple functional iPSC-derived CNS cell types and replicating complex neurodegenerative disease architectures.
RASTRUM™ is biocompatible with multiple iPSC-derived CNS cell types, enabling the generation of neural cell models with complex microenvironments.
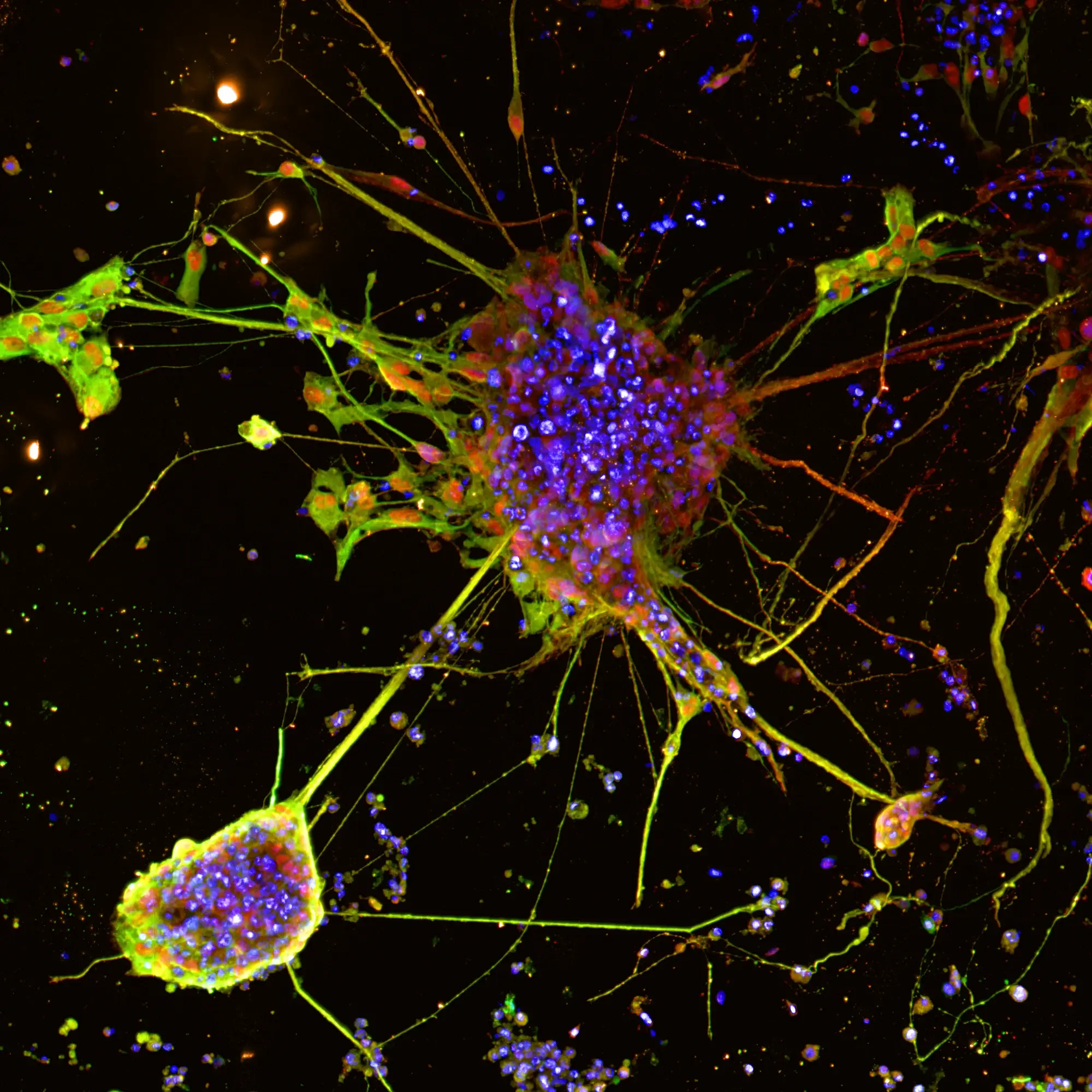
Fibroblasts play a crucial role in maintaining tissue structure, wound healing, and immune responses. Understanding fibroblast behaviour and interactions is key to unraveling the mechanisms behind various diseases, including cancer, fibrosis, and inflammatory disorders. Insights gained from fibroblast research can lead to the development of innovative therapies and interventions, making it an essential area of study in biomedical science.
RASTRUM™ offers tunable, biofunctional matrices to create physiologically relevant fibroblast microenvironments, eliciting in vivo-like phenotypes for high-throughput target discovery and phenotypic screening.
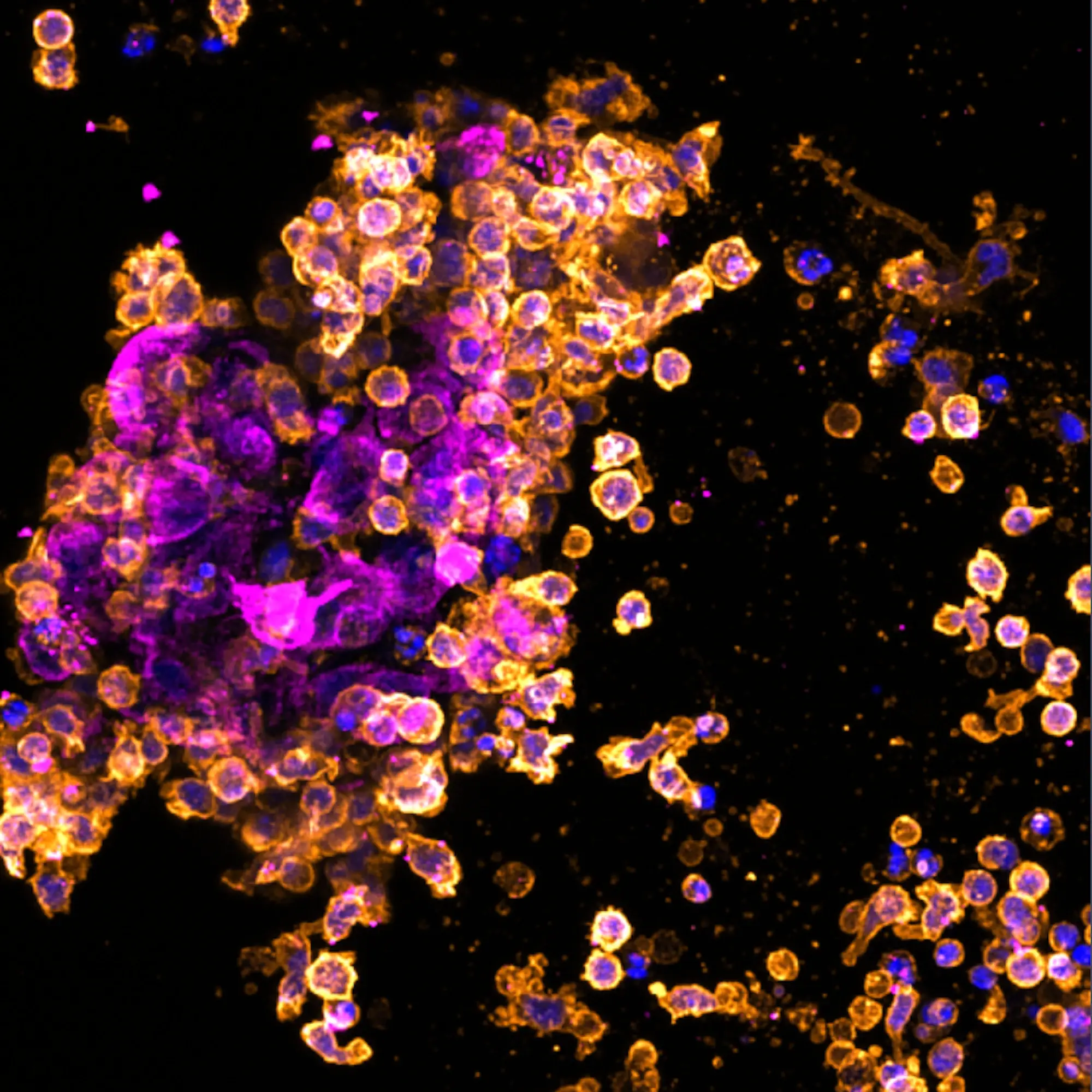
Immuno-oncology is the study of leveraging the immune system to combat cancer and has revolutionised cancer treatment. Creating physiologically relevant in vitro assays that accurately replicate the tumour microenvironment is vital for accelerating immuno-oncology research.
RASTRUM™ enables the study of tumour-immune cell interactions and the assessment of immunotherapeutic strategies in a physiologically relevant context.


