Tunable 3D cell models recapitulating the tumour microenvironment for in vitro immuno-oncology assays
Presented at European Association for Cancer Research (EACR) 2024 Congress: Innovative Cancer Science
Abstract
Introduction
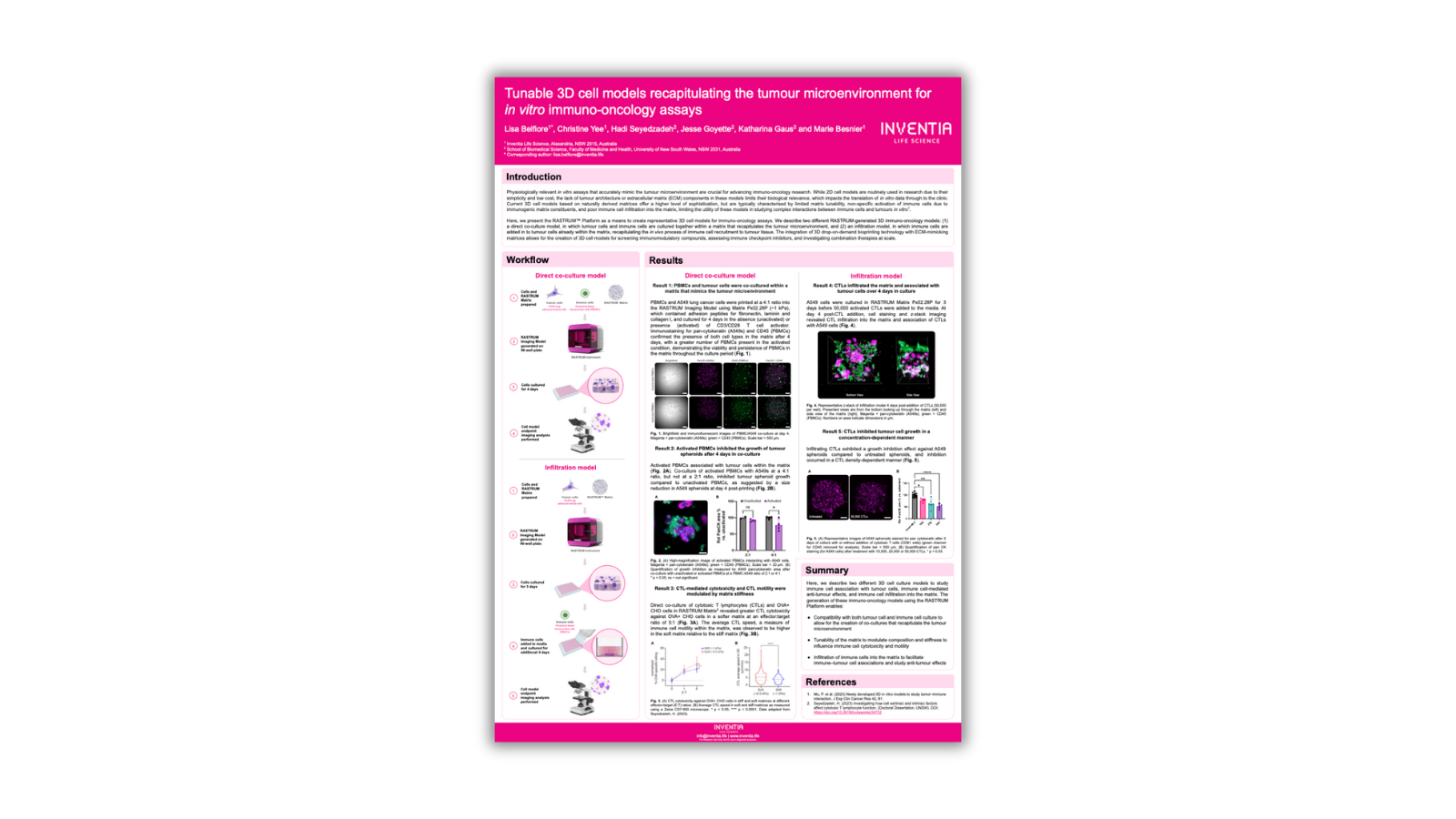
In vitro assays mimicking the tumour microenvironment are essential for immuno-oncology research. While 2D cell models are common due to simplicity and cost, they lack biological relevance, hindering translation to the clinic. Current 3D models offer sophistication but have limitations. Here, we present two 3D immuno-oncology models: direct co-culture and infiltration models. These models, generated by the RASTRUM™ Platform's drop-on-demand 3D bioprinting, allow for screening immunomodulatory compounds, assessing immune checkpoint inhibitors, and investigating combination therapies at scale.
Material and method
For the 3D cell direct co-culture, lung (A549) cancer cells and immune cells (peripheral blood mononuclear cells, PBMCs) were generated using the RASTRUM Platform (Inventia Life Science) in RASTRUM Px02.28P (~1 kPa) Matrices (Inventia Life Science). The printing design was prepared using RASTRUM Cloud Software (Inventia Life Science). 3D tumour cell models were then cultured for 4 days in the absence (unactivated) or presence (activated) of CD3/CD28 T cell activator.
For the infiltration 3D model, lung (A549) cancer cells A549 cells were cultured in RASTRUM Matrix Px02.28P for 3 days before activated cytotoxic T lymphocytes (CTLs) were added to the media. Endpoint imaging analysis performed on infiltration and co-culture of immune cells with tumour cells in 3D cell models.
Results and discussion
We used A549 cancer cells in the most physiologically relevant RASTRUM Matrix and tested their compatibility with an immuno-oncology application using PBMCs. We showed that PBMCs and tumour cells were co-cultured within a matrix that mimics the tumour microenvironment and after 4 days in co-culture, activated PBMCs inhibited the growth of tumour spheroids.
We then demonstrated infiltration of CTL within the matrix over 4 days and in addition, CTL-mediated cytotoxicity, cell growth and CTL motility were modulated by matrix stiffness.
Conclusion
In summary, we have developed two 3D cell culture models via the RASTRUM Platform for studying immune cell interactions with tumour cells and their anti-tumour effects. These models allow for co-cultures replicating the tumour microenvironment, matrix tunability to influence immune cell behaviour, and immune cell infiltration into the matrix for studying immune-tumour cell associations.